ARPCOMPLEXITY
ERC funded synergy grant defining the role of Arp2/3 complex isoforms at multiple scales of biology

The Arp2/3 complex consisting of seven subunits (Arp2, Arp3 and ARPC1–5) is conserved from yeast to man and plays an essential role in generating branched actin filament networks that provide the driving force and structural support for the physical integrity of cells, and a wide range of fundamental cellular processes including membrane trafficking and cell migration.
Interestingly, in humans and other mammals, Arp3, ARPC1 and ARPC5 exist as two different isoforms (Arp3/Arp3B, ARPC1A/ARPC1B and ARPC5/ARPC5L) that are 91, 67 and 67% identical respectively. The presence of these subunit isoforms means that the mammalian Arp2/3 complex is actually a family of 8 iso-complexes. We have previously demonstrated that these isoforms confer different properties to the Arp2/3 complex which result in differences in both the assembly and disassembly of actin networks (Abella et al., 2016 - PMID:26655834; Galloni et al., 2021 - PMID:34106209; Cao et al., 2024 PMID:39009834 and Cao et al., BioRxiv. https://doi.org/10.64898/2025.12.01.691495).
The importance of Arp2/3 complex subunit isoforms is evident from the observation that loss of human ARPC1B results in severe inflammation and immunodeficiency (PMID:28368018). In collaboration with clinicians, we have also found that loss of function mutations in ARPC5 lead to immune disease, multiple congenital anomalies, and early postnatal death (Sindram et al., 2023 - PMID:37382373). More recently, by deleting ARPC5 in the murine hematopoietic system, we uncovered why patients lacking ARPC5 develop gastrointestinal complications, immunodeficiency and are prone to fatal sepsis (Vasconcellos et al., 2025 PMID:41231985). Specifically, macrophages lacking Arpc5-, but not Arpc5l-, containing Arp2/3 complexes are unable to maintain host-microbiota homeostasis as they are deficient in phagocytosing and killing intra-cellular bacteria. In addition, the Gomes lab has demonstrated that the ARPC5 also modulates T-tubule organization in skeletal muscle by regulating cortical actin (Roman et al., 2017- PMID:28892082, Pereira, et al., 2025 BioRxiv. https://doi.org/10.1101/2024.08.13.607563)
ARPComplexity is an ERC funded synergy grant involving the labs of Michael Way (Crick Institute, London: https://www.crick.ac.uk/), Carolyn Moores (Birkbeck College, London: https://www.bbk.ac.uk/our-staff/profile/8004606/carolyn-moores) and Edgar Gomes (GIMM, Lisbon, Portugal: https://gimm.pt/lab/edgar-gomes-lab/). investigating the unique activities, interactions, cellular and physiological functions of Arp2/3 iso-complexes

This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme.
Grant agreement No 810207
ARPCOMPLEXITY PAPERS AND PREPRINTS
SPIN90 MODULATES THE ARCHITECTURE OF LAMELLIPODIAL ACTIN IN AN ARPCL DEPENDENT FASHION
LuYan Cao*, Angika Basant, Miroslav Mladenov, Naoko Kogata, Antoine Jegou, Guillaume Romet-Lemonne, Sophie Brasselet, Manos Mavrakis and Michael Way*.
* co-corresponding
BioRxiv: https://doi.org/10.64898/2025.12.01.691495
(2025)


THE ACTOMYOSIN CORTEX CONTROLS T-TUBULE REMODELING IN MUSCLE
Ana Raquel Pereira, Ana da Rosa Soares, Silvia Di Francescantonio, Tianyang Liu, Filomena A. Carvalho, Josie Liane Ferreira, Graciano Leal, Inês Faleiro, Naoko Kogata, Nuno C. Santos, Michael Way, Carolyn A. Moores and Edgar R. Gomes.
BioRxiv: https://doi.org/10.1101/2024.08.13.607563
(2025)
BRANCHED ACTIN NETWORKS MEDIATE MACROPHAGE-DEPENDENT HOST MICROBIOTA HOMEOSTASIS
Luiz Ricardo C. Vasconcellos#, Shaina Chor Mei Huang#, Alejandro Suarez-Bonnet, Simon Priestnall, Sunita Varsani-Brown, Matthew L. Winder, Kathleen Shah, Naoko Kogata, Brigitta Stockinger and Michael Way. # co-first author


ARP2/3-MEDIATED BIDIRECTIONAL ACTIN ASSEMBLY BY SPIN90 DIMERS
Tianyang Liu, Luyan Cao, Miroslav Mladenov, Guillaume Romet-Lemonne, Michael Way* and Carolyn A. Moores*
* co-corresponding
Nature Structural & Molecular Biology.
32:2262–2271. (2025)
doi:10.1038/s41594-025-01665-8
PMID: 40954369
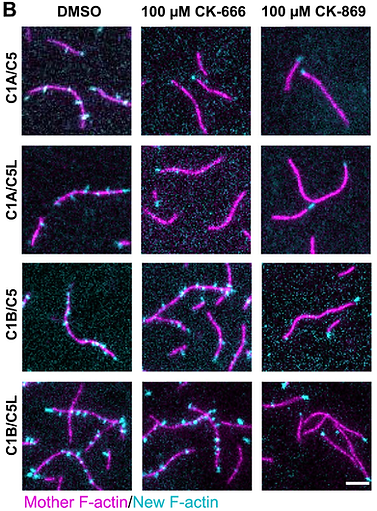
CK-666 AND CK-869 DIFFERENTIALLY INHIBIT ARP2/3 ISO-COMPLEXES
LuYan Cao*, Shaina Huang, Angika Basant, Miroslav Mladenov and Michael Way*
* co-corresponding
EMBO reports 25:3221-3239 (2024).
doi:10.1038/s44319-024-00201-x
PMID: 39009834

CORTACTIN STABILIZES ACTIN BRANCHES BY BRIDGING ACTIVATED ARP2/3 TO ITS NUCLEATED ACTIN FILAMENT
Tianyang Liu, Luyan Cao, Miroslav Mladenov, Antoine Jegou, Michael Way* and
Carolyn A. Moores*. * co-corresponding
Nature Structural & Molecular Biology
5:801-809. (2024)
doi:10.1038/s41594-023-01205-2
PMID: 38267598
ARPC5 DEFICIENCY LEADS TO SEVERE EARLY-ONSET SYSTEMIC INFLAMMATION AND MORTALITY
Elena Sindram#, Andrés Caballero-Oteyza#, Naoko Kogata#, Shaina Huang, Zahra Alizadeh, Laura Gamez, Mohammad Reza Fazlollhi, Xiao Peng, Bodo Grimbacher, Michael Way* and Michele Proietti*. # co-first author * co-corresponding
Disease Models & Mechanisms
10.1242/dmm.050145 (2023)
doi:10.1242/dmm.050145. PMID: 37382373


MICAL2 ENHANCES BRANCHED ACTIN NETWORK DISASSEMBLY BY OXIDIZING ARP3B-CONTAINING ARP2/3 COMPLEXES.
Chiara Galloni, Davide Carra, Jasmine V. G. Abella, Svend Kjær, Pavithra
Singaravelu, David J Barry, Naoko Kogata, Christophe Guérin, Laurent Blanchoin and Michael Way
J. Cell Biol. 220:e202102043. (2021)
doi:10.1083/jcb.202102043 PMID: 34106209
CRYO-EM OF HUMAN ARP2/3 COMPLEXES PROVIDES STRUCTURAL INSIGHTS INTO ACTIN NUCLEATION MODULATION BY ARPC5 ISOFORMS
Ottilie von Loeffelholz, Andrew Purkiss, Luyan Cao, Svend Kjaer, Naoko Kogata, Guillaume Romet-Lemonne, Michael Way and Carolyn A. Moores
Biology Open 9, bio054304 (2020)
doi:10.1242/bio.054304 PMID: 32661131

First synergy retreat at Crick Institute
November 2019

Zoom discussion and journal club March 2020
